
Every Safety Data Migration is different and the Sophos team with its diverse data migration experience, knows how to conquer the challenges and deliver the project with supreme quality. From Traditional data migration to enhanced E2B Import, we have experience in delivering data migrations for CROs, Biotech and Pharma companies. Some of our recent achievements include single tenant to Multi-tenant and multi-tenant to multi-tenant migrations.

- Data migration analysis (source and target)
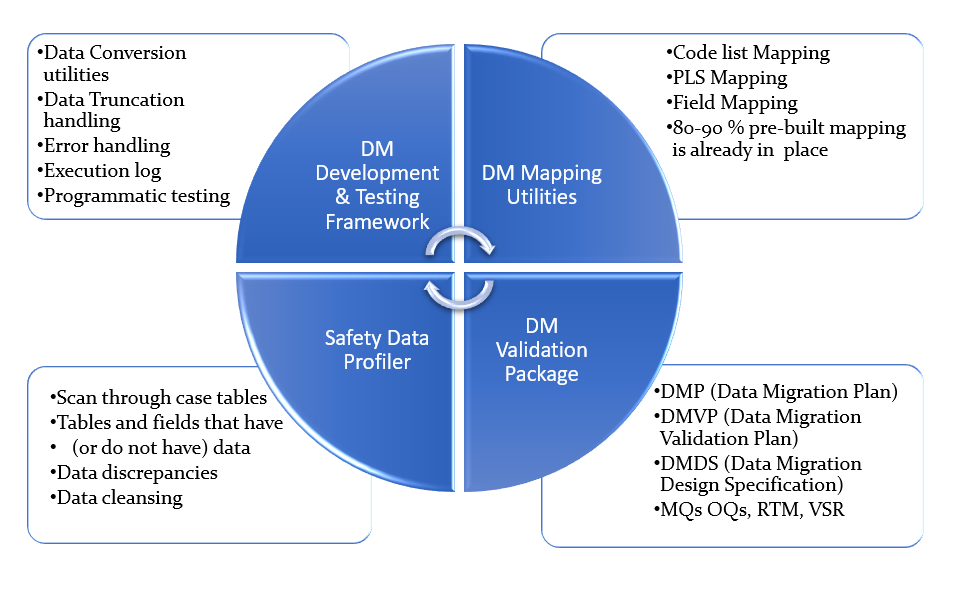
- Case volume and data profiling
- Early discovery of data discrepancies and duplicate cases
- Initial (pre-development) run
- Data migration code development (conversion area)
- Apply transformation rules
- Data cleansing, if necessary
- Duplicate case handling
- Data migration mappings
- Dry runs
- Error handling & process log
- Error logging per case basis
- Remediate errors via full/partial run
- Validation
- Automated (programmatic) testing ensures 100% testing of mappings
- Reports (MedWatch, CIOMS, E2B etc.) comparison ensures if mappings were correct


- ARISg/ARISj to Argus/ArgusJ
- Argus to Argus
- Argus to ARISg
- AERS/Emperica Trace to Argus
- Perceive to ArgusJ
- Home grown systems, Excel, flat files, XML to Argus

- Pre-defined high quality data mappings (Any Safety system to Argus)
- Data cleansing for data integrity and consistency
- High quality Sophos Validation Package that helps customers comply with 21 CFR Part 11 requirements
- Impact analysis and action plan for downstream applications
- High case volume data migration having 2.5 Million cases
- High complexity data migration where cases are spread across different systems globally requiring reconciliation and merge into single case
- Efficient planning and execution to shorten downtime
- Migration of custom reports


